- India’s efforts to achieve 1.5 billion COVID-19 vaccinations: a narrative review
-
Kapil Singh, Ashwani Verma, Monisha Lakshminarayan
-
Osong Public Health Res Perspect. 2022;13(5):316-327. Published online October 14, 2022
-
DOI: https://doi.org/10.24171/j.phrp.2022.0104
-
-
3,749
View
-
91
Download
-
8
Web of Science
-
12
Crossref
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
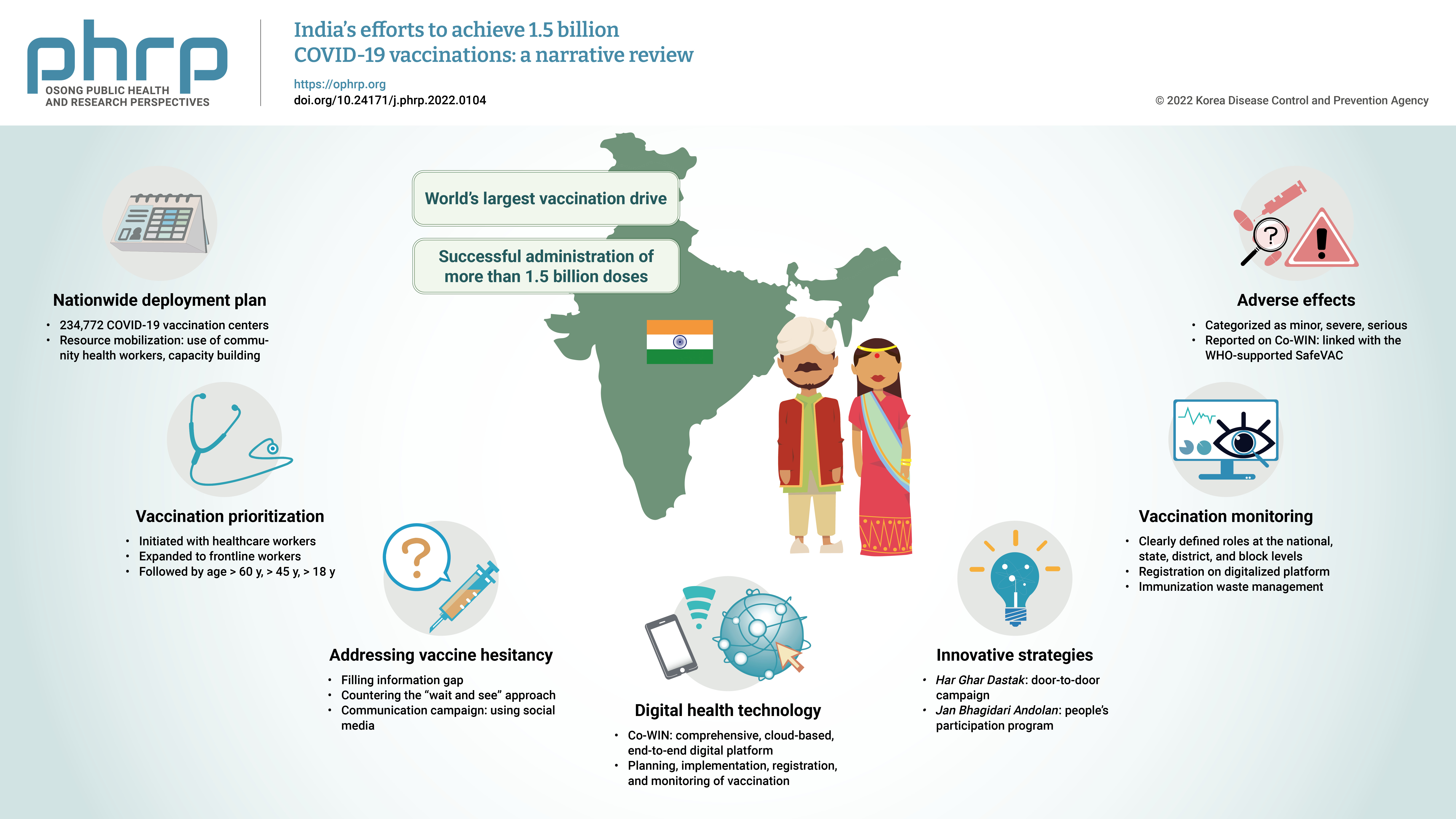 - The initial case of coronavirus disease 2019 (COVID-19) in India was reported on January 30, 2020, and subsequently, the number of COVID-19-infected patients surged during the first wave of April 2020 and the second wave in the same month of 2021. The government of India imposed a strict nationwide lockdown in April 2020 and extended it until May 2020. The second wave of COVID-19 in India overwhelmed the country’s health facilities and exhausted its medical and paramedical workforce. This narrative review was conducted with the aim of summarizing the evidence drawn from policy documents of governmental and non-governmental organizations, as well as capturing India's COVID-19 vaccination efforts. The findings from this review cover the Indian government's vaccination initiatives, which ranged from steps taken to combat vaccine hesitancy to vaccination roadmaps, deployment plans, the use of digital health technology, vaccination monitoring, adverse effects, and innovative strategies such as Har Ghar Dastak and Jan Bhagidari Andolan (people’s participation). These efforts collectively culminated in the successful administration of more than 1.8 billion doses of COVID-19 vaccines in India. This review also provides insights into other countries’ responses to COVID-19 and guidance for future pandemics.
-
Citations
Citations to this article as recorded by  - Digital health technology used in emergency large-scale vaccination campaigns in low- and middle-income countries: a narrative review for improved pandemic preparedness
Paula Mc Kenna, Lindsay A. Broadfield, Annik Willems, Serge Masyn, Theresa Pattery, Ruxandra Draghia-Akli
Expert Review of Vaccines.2023; 22(1): 243. CrossRef - Media Reporting Relating to COVID-19 Vaccination as a Driver of Vaccine Hesitancy Prior to the Second Wave of the COVID-19 Pandemic in India: A Content Analysis of Newspaper and Digital Media Reports
Saurav Basu, Himanshi Sharma
Cureus.2023;[Epub] CrossRef - An assessment of the strategy and status of COVID-19 vaccination in India
Sneh Lata Gupta, Surbhi Goswami, Ananya Anand, Namrata Naman, Priya Kumari, Priyanka Sharma, Rishi K. Jaiswal
Immunologic Research.2023; 71(4): 565. CrossRef - Development of a Choice-framework for Covid vaccines in India using a multi-criteria decision analysis approach
Tarun K. George, Nayana P. Nair, Awnish Kumar Singh, A. Dilesh Kumar, Arup Deb Roy, Varshini Neethi Mohan, Gagandeep Kang
Vaccine.2023; 41(25): 3755. CrossRef - COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India
Nandini Sharma, Saurav Basu, Heena Lalwani, Shivani Rao, Mansi Malik, Sandeep Garg, Rahul Shrivastava, Mongjam Meghachandra Singh
Vaccines.2023; 11(7): 1177. CrossRef - Review of the unmet medical need for vaccination in adults with immunocompromising conditions: An Indian perspective
Ashok Vaid, Neha Rastogi, T. Mark Doherty, Peter San Martin, Yashpal Chugh
Human Vaccines & Immunotherapeutics.2023;[Epub] CrossRef - Translating the COVID-19 experience in widening the HPV vaccination campaign for cervical cancer in India
Aruni Ghose, Anisha Agarwal, Bhawna Sirohi, Shona Nag, Linus Chuang, Swarupa Mitra
Gynecologic Oncology Reports.2023; 48: 101247. CrossRef - Symptomatic prevalence of covid-19 in vaccinated and non-vaccinated population
Jay Bhupesh Pandya, Nirali Milind Shethia, Divya Bangera, Shailaja Gada Saxena
IP International Journal of Medical Microbiology a.2023; 9(2): 110. CrossRef - Active surveillance of adverse events following COVID-19 vaccines in a tertiary care hospital
Naveena Mary Cherian, Dravya Anna Durai, Muhammed Jaisel, Divyansh Sharma, Juny Sebastian, Chetak Kadabasal Basavaraja, Merrin Mathew
Therapeutic Advances in Vaccines and Immunotherapy.2023;[Epub] CrossRef - Effectiveness of ayurvedic formulation, NAOQ19 along with standard care in the treatment of mild-moderate COVID-19 patients: A double blind, randomized, placebo-controlled, multicentric trial
Pankaj Bhardwaj, Kalaiselvan Ganapathy, Monika Pathania, K.H. Naveen, Jaykaran Charan, Siddhartha Dutta, Ravisekhar Gadepalli, Srikanth Srinivasan, Manoj Kumar Gupta, Akhil D. Goel, Naresh Midha, Bharat Kumar, Meenakshi Sharma, Praveen Sharma, Mithu Baner
Journal of Ayurveda and Integrative Medicine.2023; 14(6): 100778. CrossRef - Balancing Routine and Pandemic: The Synergy of India’s Universal Immunization Program and COVID-19 Vaccination Program
Pawan Kumar, Ashish Birendra Chakraborty, Suhas Dhandore, Pritu Dhalaria, Ajeet Kumar Singh, Disha Agarwal, Kapil Singh, Pretty Priyadarshini, Paras Jain, Vidushi Bahl, Gunjan Taneja
Vaccines.2023; 11(12): 1776. CrossRef - Unveiling vaccine safety: a narrative review of pharmacovigilance in India's COVID-19 vaccination
Megha Hegde, Saurav Raj, Dhananjay Tikadar, Sanatkumar B Nyamagoud
Monaldi Archives for Chest Disease.2023;[Epub] CrossRef
|